Geographical Distribution in Africa
Geographical Distribution of Purple witchweed in Africa (red marked). Updated on 11 July 2019. Source CABI
General Information on Pest and Damage
Introduction
The purple witchweed Striga hermonthica threatens the lives of over 100 million people in Africa and infest about 40% of arable land in the savanna region, causing an estimated annual loss of $ 7 to 13 billion (www.icipe.org). It is almost certainly responsible for more crop loss in Africa than any other individual weed species. Over 5 million ha of crops - mainly sorghum, millets and maize - are affected in six countries of West Africa alone, possibly 10 million ha in Africa as a whole. One plant of S. hermonthica per host plant is estimated to cause approximately 5% loss of yield (Parker and Riches, 1993) and high infestations can cause total crop failure. Overall yield losses are estimated at 21% of all sorghum in northern Ghana, 10% of all cereals in Nigeria, 8% in Gambia and 6% in Benin. Other countries seriously affected include Cameroon, Cote d'Ivoire, Burkina Faso, Niger, Mali, Senegal, Togo, Sudan, Ethiopia, Kenya, Uganda and Tanzania.
The damaging effect of S. hermonthica on the host plant derives not only from the direct loss of water, minerals, nitrogen and carbohydrate, but from a disturbance of the host photosynthetic efficiency and a profound change in the root/shoot balance of the host, leading to stimulation of the root system and stunting of the shoot. Young Striga seedlings are completely parasitic on the host while they are below the soil level and, at this stage, cause maximum damage to the host.
As S. hermonthica occurs mainly under conditions of low fertility. It is also associated with farming systems in Africa in which farmers have few resources and very few options in terms of control measures.
Symptoms
S. hermonthica causes characteristic yellowish blotches in the foliage about 1 cm long by 0.5 cm wide. In later stages whole leaves may wilt, become chlorotic and die. Stems are shortened, though leaf number may not be reduced. Inflorescence development is delayed or prevented. Root systems, at least in early stages, may be stimulated, and haustoria 1-2 mm across appear like nodules.
Host range
The natural host range of S. hermonthica is normally limited to Gramineae (Poaceae), but weak attachment to groundnut, cowpea, lablab and soyabean was obtained in pots and there have been unconfirmed reports of infestation of groundnut and sesamum fields in West Africa. Apart from the wild hosts listed above, S. hermonthica is occasionally observed on crowfoot grass (Dactyloctenium aegyptium), Panicum walense, goose grass (Eleusine indica), rice grass paspalum (Paspalum scrobiculatum), Pennisetum violaceum and on Cynodon, Cymbopogon, Ophiuros and Brachiaria spp. Individual biotypes may have a narrower host range than the species. In particular, there are forms which attack sorghum but not pearl millet and vice versa.
Affected plant stages
Flowering stage, fruiting stage, pre-emergence, seedling stage and vegetative growing stage.
Affected plant parts
Leaves, stems and whole plant.
Leaves: abnormal patterns; yellowed or dead.
Stems: abnormal growth.
Whole plant: dwarfing; early senescence
Biology and Ecology of Purple Witchweed
The biology and ecology of S. hermonthica are described in detail by Parker and Riches (1993). It is an obligate hemi-parasite, with green foliage capable of photosynthesis and can at least partially support its own growth once established. Its minute seeds (about 0.2 x 0.3 mm) have inadequate reserves to establish without a host. Seeds are produced in enormous numbers and they are generally dispersed by wind, water, livestock and man.
Relatively high temperatures, 30-35°C, are optimal for germination and for growth. Dry conditions of soil and air are most favourable, and S. hermonthica rarely occurs in irrigated cereals, though wet conditions can be tolerated for short periods. Neither soil type nor pH is critical for its growth - S. hermonthica occurs on almost all soil types from sandy acidic to alkaline clay soils, as in Sudan.
Habitat
S. hermonthica, as most other Striga species, is associated with low-fertility soils, especially those low in nitrogen. Unlike Striga asiatica it occurs not only on light, sandy soils but also on heavy clays and even on vertisols. It is also favoured by low soil moisture, and rarely occurs on irrigated soils, but can tolerate abundant moisture for short periods. It is a plant of African savanna, almost invariably associated with cereal cropping and relatively uncommon in natural vegetation.
S. hermonthica is a herbaceous annual plant 30-100 cm high, the most robust forms occurring in Sudan and Ethiopia. Larger plants may be much branched. The root system is weak with little or no ability to absorb materials from the soil, but branches develop from lower nodes of the plant, ramifying and developing attachments on contact with other host roots.
Pest and Disease Management
Pest and disease management: General illustration of the concept of Infonet-biovision
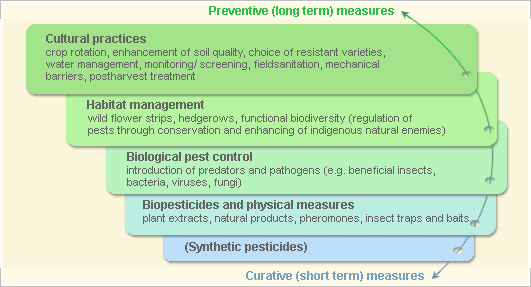
These illustration shows the methods promoted on infonet-biovision. The methods shown at the top have a long-term effect, while methods shown at the bottom have a short-term effect. In organic farming systems, methods with a long-term effect are the basis of crop production and should be used with preference. On the other hand methods with a short-term effect should be used in emergencies only. On infonet we do not promote synthethic pesticides.
Cultural practices
Detection and inspection methods
Infestation of a cereal crop by S. hermonthica may be apparent before emergence from the soil, by the chlorotic blotches on the crop foliage. Uprooting may confirm the presence of the haustoria and young parasite seedlings on the root.
Resistant/ tolerant varieties
No completely immune cereal varieties have yet been developed, but many sorghum varieties show high levels of resistance, at least under local conditions. Selection and breeding programmes in India and Africa have led to the development and release of many lines with at least reduced susceptibility, and these may be valuable as components of an integrated control approach. However, traditional varieties in Striga-infested areas often show relatively high tolerance, and these may yield well in spite of heavy infestation. Identified resistant cultivars of sorghum include 'N-13' from India, 'Framida' from South Africa, 'Serena', 'SAR 29' from Tanzania. Among millets the following cultivars are considered resistant in Tanzania: 'Buruma', 'Shibe', 'Okoa' and 'Serere 17'.
In maize there are no effectively resistant varieties, though some show partial resistance, such as 'Katumani' in Kenya, and some more tolerant lines have been developed by the International Institute for Tropical Agriculture. Work is now in progress to transfer high-level resistance into maize from wild relatives including Zea diploperennis.
Little progress has been possible with the out-crossing pearl millet, but there are promising results from work in progress to select and develop rice varieties with resistance to Striga species.
Characteristics/weaknesses in S. hermonthica which may be exploited in cultural control measures include the following:
- Dependence on a susceptible host for establishment: Crop rotation avoiding a susceptible cereal will prevent new seeding and allow decline of the soil seed bank. In some areas, there may be alternative cereals which are not attacked (e.g. sorghum in a millet-growing area, or vice versa). Among the non-cereal crops, many are known to exude germination stimulant, though they cannot be parasitised. These trap crops, such as cotton, groundnut, cowpea, sunflower and soyabean, are especially beneficial in causing suicidal germination and accelerating a decline in the soil seed bank. But they need to be sown at a time when Striga germination is likely to be high, usually early in the rainy season, before the onset of any secondary dormancy. Some of the catch crops are susceptible cereals which may be grown at the beginning of the season or in short rains prior to the main season, to stimulate germination of the Striga. However, they need to be destroyed before the weed can mature and set seed.
- Preference for low nitrogen: Additional nitrogen fertilizer usually reduces Striga incidence, though not always, especially when applied as a single dose. However, improved soil fertility is a vital key to long-term control, whether by organic, inorganic or green manuring, rotation with legumes, or agroforestry techniques involving mulching.
- Preference for dry conditions: Irrigation is rarely an option, but moisture conservation techniques may be beneficial. Any means of raising humidity will reduce Striga transpiration and its ability to draw nutrition from the host. Hence leafy crop varieties, dense, uniform planting and intercropping with legumes, all tend to suppress the weed.
None of the methods described above will, alone, provide complete control, and without complete control there is the certainty that surviving Strigaplants will mature and replenish the soil seed bank. It is therefore essential that manual and mechanical methods are used to destroy surviving Strigaplants. Hand-pulling is the most common traditional technique, though a late hoeing or ridging may also be effective.
ICIPE developed a habitat management strategy for effective control of Striga in cereal-based farming systems. It involves planting desmodium legume (Desmodium uncinatum) intercrop in maize fields. Roots of desmodium secrete chemicals (isoflavones) that stimulate Striga seed germination and also inhibit attachment of Striga to maize roots thereby causing suicidal germination of Striga seed and reducing its seed bank in the soil. The legume also maintains soil stability and improves soil fertility through nitrogen fixation. In addition it serves as a highly nutritious animal feed. This habitat management strategy is part of an integrated approach called "push and pull" for control of both maize stemborers and Striga (www.icipe.org). For more information on push-pull click here
For more information on weeds click here
Biological pest control
Biological Control
Other potentially useful organisms for Striga management include the following: a) the butterfly Precis (=Junonia) species whose larvae feed on leaves, buds and capsules of many Striga species and b) a range of fungal diseases including Fusarium equiseti that causes girdling of the stem, abortion of seed capsules or wilting and death of young Striga plants.
Integrated control
As virtually none of the treatments described above is likely to achieve complete control, integration of one or more is essential for any substantial reduction of the problem. Furthermore, such integrated treatments will almost certainly need to be repeated over a number of years for long-term control. Parker and Riches (1993) propose a range of programmes depending on the initial density of the problem, involving various combinations of rotation, varietal selection, soil fertility enhancement and intercropping with legumes, supplemented in all cases by hand-pulling.
Information Source Links
- Crop Protection Compendium, 2005 Edition. © CAB International, Wallingford, UK, 2005 www.cabi.org
- ICIPE Biennial Scientific Report (2004-2005). Habitat management strategies for control of stemborers and Striga weed in cereal-based farming systems. pp. 40-58. www.icipe.org
- Mbwaga, A. (1996). Fahamu gugu chawi (Striga) la mimea yako ya nafaka. Ministry of Agriculture, Department of Research and Training, Plant Protection Programme, Tanzania.
- Parker, C., Riches, C.R. (1993). Parasitic weeds of the world: Biology and Control. Wallingford, UK: CAB International.
- Ramaiah, K.V., Parker, C., Vasudeva Rao, M.J., and Musselman, L.J. (1983). Striga Identification and Control Handbook. Information Bulletin No. 15. Patancheru, A.P., India: International Crops Research Institute for Semi-Arid Tropics (ICRISAT)
- Terry, P.J. and Michieka, R.W. (1987). Common weeds of East Africa. Food and Agriculture Organization of the United Nations. ISBN: 92 5 002426 6
